These laboratory testing policies apply to Dual Special Needs Populations (DSNP) plans. These CareSource policies are published by Avalon Healthcare Solutions on behalf of CareSource.
These policies offer guidance on determination of medical necessity and appropriateness of care for approved benefits. Benefit determinations and coverage decisions are subject to all the terms and conditions of CareSource including eligibility, definitions, specific inclusions or exclusions, and applicable state or federal laws. Policies are considered guidelines and are not intended to infer benefits or coverage for a specific member.
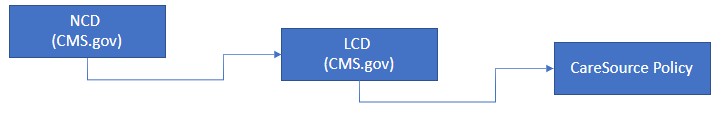
Policy Hierarchy
Decision making for Dual Special Needs Populations (DSNP) plans are based upon the following hierarchy:
- F2019: Flow Cytometry – Effective Date: 10-01-2023
- F2019: Flow Cytometry – Effective Date: 02-01-2025
- G2002: Cervical Cancer Screening – Effective Date: 03-01-2024
- G2002: Cervical Cancer Screening – Effective Date: 02-01-2025
- G2005: Vitamin D Testing – Effective Date: 05-01-2024
- G2005: Vitamin D Testing – Effective Date: 05-01-2025
- G2006: Diabetes Mellitus Testing – Effective Date: 08-01-2024
- G2006: Diabetes Mellitus Testing – Effective Date: 05-01-2025
- G2007: Prostate Biopsy Specimen Analysis – Effective Date: 03-01-2024
- G2007: Prostate Biopsy Specimen Analysis – Effective Date: 02-01-2025
- G2008: Prostate Specific Antigen (PSA) Testing – Effective Date: 10-01-2023
- G2008: Prostate Specific Antigen (PSA) Testing – Effective Date: 08-01-2024
- G2009: Preventive Screening in Adults – Effective Date: 08-01-2023 to 09-30-2023
- G2011: Diagnostic Testing of Iron Homeostasis & Metabolism – Effective Date: 05-01-2024
- G2011: Diagnostic Testing of Iron Homeostasis & Metabolism – Effective Date: 05-01-2025
- G2013: Testosterone – Effective Date: 05-01-2024
- G2013: Testosterone – Effective Date: 05-01-2025
- G2014: Vitamin B12 and Methylmalonic Acid Testing – Effective Date: 05-01-2024
- G2014: Vitamin B12 and Methylmalonic Acid Testing – Effective Date: 05-01-2025
- G2022: Biomarker Testing for Autoimmune Rheumatic Disease – Effective Date: 10-01-2023
- G2022: Biomarker Testing for Autoimmune Rheumatic Disease – Effective Date: 08-01-2024
- G2023: Pre Operative Testing – Effective Date: 01-01-2022 to 04-30-2022
- G2031: Allergen Testing – Effective Date: 10-01-2023
- G2031: Allergen Testing – Effective Date: 08-01-2024
- G2035: Prenatal Screening (Nongenetic) – Effective Date: 12-01-2023
- G2035: Prenatal Screening (Nongenetic) – Effective Date: 02-01-2025
- G2036: Hepatitis Testing – Effective Date: 05-01-2024
- G2036: Hepatitis Testing – Effective Date: 02-01-2025
- G2042: Pediatric Preventive Screening – Effective Date: 10-01-2023
- G2042: Pediatric Preventive Screening – Effective Date: 08-01-2024
- G2043: Celiac Disease Testing – Effective Date: 03-01-2024
- G2043: Celiac Disease Testing – Effective Date: 02-01-2025
- G2044: Helicobacter pylori Testing – Effective Date: 10-01-2023
- G2044: Helicobacter pylori Testing – Effective Date: 08-01-2024
- G2045: Thyroid Disease Testing – Effective Date: 10-01-23
- G2045: Thyroid Disease Testing – Effective Date: 02-01-2025
- G2048: Biochemical Markers of Alzheimer Disease and Dementia – Effective Date: 03-01-2024
- G2048: Biochemical Markers of Alzheimer Disease and Dementia – Effective Date: 02-01-2025
- G2050: Cardiovascular Disease Risk Assessment – Effective Date: 08-01-2024
- G2050: Cardiovascular Disease Risk Assessment – Effective Date: 02-01-2025
- G2051: Bone Turnover Markers Testing – Effective Date: 03-01-2024
- G2051: Bone Turnover Markers Testing – Effective Date: 02-01-2025
- G2055: Prenatal Screening for Fetal Aneuploidy – Effective Date: 11-01-2022 to 04-30-2023
- G2056: Diagnosis of Idiopathic Environmental Intolerance – Effective Date: 08-01-2024
- G2056: Diagnosis of Idiopathic Environmental Intolerance – Effective Date: 05-01-2025
- G2059: Epithelial Cell Cytology in Breast Cancer Risk Assessment – Effective Date: 03-01-2024
- G2059: Epithelial Cell Cytology in Breast Cancer Risk Assessment – Effective Date: 02-01-2025
- G2060: Fecal Analysis in the Diagnosis of Intestinal Dysbiosis and Fecal Microbiota Transplant Testing – Effective Date: 03-01-2024
- G2060: Fecal Analysis in the Diagnosis of Intestinal Dysbiosis and Fecal Microbiota Transplant Testing – Effective Date: 02-01-2025
- G2061: Fecal Calprotectin Testing in Adults – Effective Date: 03-01-2024
- G2061: Fecal Calprotectin Testing in Adults – Effective Date: 02-01-2025
- G2063: Testing for Diagnosis of Active or Latent Tuberculosis – Effective Date: 03-01-2024
- G2063: Testing for Diagnosis of Active or Latent Tuberculosis – Effective Date: 02-01-2025
- G2098: Immune Cell Function Assay – Effective Date: 03-01-2024
- G2098: Immune Cell Function Assay – Effective Date: 05-01-2025
- G2099: Intracellular Micronutrient Analysis – Effective Date: 10-01-2023
- G2099: Intracellular Micronutrient Analysis – Effective Date: 08-01-2024
- G2100: In Vitro Chemoresistance and Chemosensitivity Assays – Effective Date: 03-01-2024
- G2100: In Vitro Chemoresistance and Chemosensitivity Assays – Effective Date: 02-01-2025
- G2105: Immunopharmacologic Monitoring of Therapeutic Serum Antibodies – Effective Date: 03-01-2024
- G2105: Immunopharmacologic Monitoring of Therapeutic Serum Antibodies – Effective Date: 02-01-2025
- G2107: Measurement of Thromboxane Metabolites for ASA Resistance – Effective Date: 03-01-2024 to 12-31-2024
- G2110: Serum Testing for Hepatic Fibrosis in the Evaluation and Monitoring of Chronic Liver Disease – Effective Date: 03-01-2024
- G2110: Serum Testing for Hepatic Fibrosis in the Evaluation and Monitoring of Chronic Liver Disease – Effective Date: 02-01-2025
- G2113: Oral Cancer Screening and Testing – Effective Date: 12-01-2023
- G2113: Oral Cancer Screening and Testing – Effective Date: 02-01-2025
- G2115: Metabolite Markers of Thiopurines Testing – Effective Date: 03-01-2024
- G2115: Metabolite Markers of Thiopurines Testing – Effective Date: 02-01-2025
- G2119: Diagnostic Testing of Influenza – Effective Date: 03-01-2024
- G2119: Diagnostic Testing of Influenza – Effective Date: 02-01-2025
- G2120: Salivary Hormone Testing – Effective Date: 10-01-2023
- G2120: Salivary Hormone Testing – Effective Date: 08-01-2024
- G2121: Laboratory Testing for the Diagnosis of Inflammatory Bowel Disease – Effective Date: 03-01-2024
- G2121: Laboratory Testing for the Diagnosis of Inflammatory Bowel Disease – Effective Date: 02-01-2025
- G2123: Serum Biomarker Testing for Multiple Sclerosis and Related Neurologic Diseases – Effective Date: 03-01-2024
- G2123: Serum Biomarker Testing for Multiple Sclerosis and Related Neurologic Diseases – Effective Date: 02-01-2025
- G2124: Serum Tumor Markers for Malignancies – Effective Date: 05-01-2024
- G2124: Serum Tumor Markers for Malignancies – Effective Date: 02-01-2025
- G2125: Urinary Tumor Markers for Bladder Cancer – Effective Date: 10-01-2023
- G2125: Urinary Tumor Markers for Bladder Cancer – Effective Date: 08-01-2024
- G2127: Vectra DA Blood Test for Rheumatoid Arthritis – Effective Date: 11-01-2022 to 09-30-2023
- G2130: ST2 Assay for Chronic Heart Failure – Effective Date: 01-01-2023 to 11-30-2023
- G2132: Erectile Dysfunction – Effective Date: 01-01-2023 to 09-30-2023
- G2133: ZIKA Virus Risk Assessment – Effective Date: 11-01-2022 to 11-30-2023
- G2138: Evaluation of Dry Eyes – Effective Date: 10-01-2023
- G2138: Evaluation of Dry Eyes – Effective Date: 08-01-2024
- G2143: Lyme Disease Testing – Effective Date: 10-01-2023
- G2143: Lyme Disease Testing – Effective Date: 08-01-2024
- G2149: Pathogen Panel Testing; efd 02-01-2025
- G2149: Pathogen Panel Testing – Effective Date: 05-01-2025
- G2150: Biomarkers for Myocardial Infarction and Chronic Heart Failure – Effective Date: 03-01-2024
- G2150: Biomarkers for Myocardial Infarction and Chronic Heart Failure – Effective Date: 02-01-2025
- G2151: Serum Testing For Evidence of Mild Traumatic Brain Injury – Effective Date: 02-01-2025
- G2153: Pancreatic Enzyme Testing for Acute Pancreatitis – Effective Date: 12-01-2023
- G2153: Pancreatic Enzyme Testing for Acute Pancreatitis – Effective Date: 02-01-2025
- G2154: Folate Testing – Effective Date: 02-01-2025
- G2154: Folate Testing – Effective Date: 05-01-2025
- G2155: General Inflammation Testing – Effective Date: 12-01-2023
- G2155: General Inflammation Testing – Effective Date: 02-01-2025
- G2156: Urine Culture Testing for Bacteria – Effective Date: 12-01-2023
- G2156: Urine Culture Testing for Bacteria – Effective Date: 02-01-2025
- G2157: Diagnostic Testing of Common Sexually Transmitted Infections – Effective Date: 03-01-2024
- G2157: Diagnostic Testing of Common Sexually Transmitted Infections – Effective Date: 02-01-2025
- G2158: Testing for Vector-Borne Infections – Effective Date: 12-01-2023
- G2158: Testing for Vector-Borne Infections – Effective Date: 02-01-2025
- G2159: B-Hemolytic Streptococcus Testing – Effective Date: 03-01-2024
- G2159: B-Hemolytic Streptococcus Testing – Effective Date: 02-01-2025
- G2161: Hormonal Testing in Adult Females – Effective Date: 01-01-2022 to 05-31-2022
- G2164: Parathyroid Hormone, Phosphorus, Calcium, and Magnesium Testing – Effective Date: 10-01-2023
- G2164: Parathyroid Hormone, Phosphorus, Calcium, and Magnesium Testing – Effective Date: 08-01-2024
- G2173: Gamma-glutamyl Transferase – Effective Date: 12-01-2023
- G2173: Gamma-glutamyl Transferase Testing in Adults – Effective Date: 02-01-2025
- G2174: Coronavirus Testing in the Outpatient Setting – Effective Date: 05-01-2024
- G2174: Coronavirus Testing in the Outpatient Setting – Effective Date: 05-01-2025
- G2181: Colorectal Cancer Screening – Effective Date: 08-01-2024
- G2181: Colorectal Cancer Screening – Effective Date: 04-01-2025
- M2041: Venous and Arterial Thrombosis Risk Testing – Effective Date: 10-01-2023
- M2041: Venous and Arterial Thrombosis Risk Testing – Effective Date: 02-01-2025
- M2057: Diagnosis of Vaginitis – Effective Date: 05-01-2024
- M2057: Diagnosis of Vaginitis – Effective Date: 08-01-2024
- M2058: Genetic Testing for Adolescent Idiopathic Scoliosis – Effective Date: 01-01-2023 to 09-30-2023
- M2067: Therapeutic Drug Monitoring for 5-Fluorouracil – Effective Date: 05-01-2025
- M2068: Testing for Alpha-1 Antitrypsin Deficiency – Effective Date: 12-01-2023
- M2068: Testing for Alpha-1 Antitrypsin Deficiency – Effective Date: 02-01-2025
- M2093: HIV Genotyping and Phenotyping – Effective Date: 01-01-2023 to 09-30-2023
- M2097: Identification of Microorganisms Using Nucleic Acid Probes – Effective Date: 12-01-2023
- M2097: Identification of Microorganisms Using Nucleic Acid Probes – Effective Date: 02-01-2025
- M2112: Nerve Fiber Density Testing – Effective Date: 03-01-2024
- M2112: Nerve Fiber Density Testing – Effective Date: 02-01-2025
- M2116: Human Immunodeficiency Virus (HIV) – Effective Date: 10-01-2023
- M2116: Human Immunodeficiency Virus (HIV) – Effective Date: 08-01-2024
- M2136: DNA Ploidy Cell Cycle Analysis – Effective Date: 01-01-2022 to 09-30-2022
- M2141: Testing of Homocysteine Metabolism-Related Conditions – Effective Date: 05-01-2024
- M2141: Testing of Homocysteine Metabolism-Related Conditions – Effective Date: 05-01-2025
- M2172: Onychomycosis Testing – Effective Date: 10-01-2023
- M2172: Onychomycosis Testing – Effective Date: 08-01-2024
- M2176: Testing for Autism Spectrum Disorder and Developmental Delay – Effective Date: 05-01-2024
- M2176: Testing for Developmental Delay – Effective Date: 05-01-2025
- P2018: Immunohistochemistry – Effective Date: 05-01-2024
- P2018: Immunohistochemistry – Effective Date: 05-01-2025
- R2162: Laboratory Procedures Reimbursement Policy – Effective Date: 08-01-2022
- R2162: Laboratory Procedures Reimbursement Policy – Effective Date: 05-01-2025
- T2015: Prescription Medication and Illicit Drug Testing in the Outpatient Setting – Effective Date: 12-01-2023
- T2015: Prescription Medication and Illicit Drug Testing in the Outpatient Setting – Effective Date: 08-01-2024
