CONTENTS
LAB COALITION AUGUST 26 INVITATION
INVITATION TO SEPTEMBER AVALON WEBINAR: Delivering Value-Driven Care Using Lab Test Results
EXCERPT FROM THE 2021 AVALON LAB TREND REPORT
AVALON LABORATORY NETWORK COVID-19 CAPACITY & TURNAROUND TIME REPORT*
TESTOSTERONE TESTING: IS IT ACCURATE & DRIVING THE RIGHT CARE?
On July 20th, 2021, Avalon Healthcare Solutions in collaboration with PATH (The Partnership for the Accurate Testing of Hormones) presented on improving patient care through the accurate and reliable testing of hormones.
Rahul Singal, M.D., the Chief Medical Officer at Avalon, began the presentation by introducing the landscape of testing to-date. For the past 50 years, the CDC has worked with industry and commercial healthcare to standardize a variety of tests that impact chronic condition management. For some time, the focus was cholesterol, as science was just beginning to uncover the relationship between high cholesterol and heart disease. Around 25 years ago, the focus moved to diabetes and hemoglobin A1C.
Past Challenges in Variability of Testing Results: A Case Study
Using hemoglobin A1C as an example, the figure below establishes how reference ranges for testing were once inconsistent and unreliable. If a physician presented the test values of a patient and did not provide the reference range, the results were difficult to compare. Variability was dramatic. The CDC was involved in the standardization of hemoglobin A1C ranges to fix this problem. Since reference ranges have been established, now you can easily treat patients as a single number (test value) will carry over from lab to lab.
Avalon has joined the effort to advance standardized assays for testosterone in partnership with PATH. We are uniquely positioned as an industry expert in the laboratory benefits space to assist in this important endeavor.

A New Focus on Hormones
Avalon welcomed two prestigious guests to discuss hormone testing: Hubert W. Vesper, Ph.D. and Alvin W. Matsumoto, M.D.
Dr. Vesper is an analytical chemist by training and a co-founder of PATH, as well as the Director of Clinical Standardization Programs, and Chief of the Protein Biomarker Laboratory and Lipid Reference Laboratory at the CDC. Dr. Matsumoto is the lead scientist of PATH and a Professor Emeritus at the University of Washington.
They presented on the dual PATH mission: (1) to advance the development of standardized hormone assays that are traceable to a single higher order reference material and method and (2) to advocate for the universal adoption of these assays in medical practice and research.
How did this mission come about? In 2008, the Endocrine Society approached the CDC for help because hormone lab tests were not considered accurate and reliable. In response, the CDC convened a group to discuss the testing of hormones. The mission of this group was to provide accurate and comparable (that is, standardized) tests for consistent and reliable diagnosis by clinicians.
The Challenge: Manufacturers’ Assays Show Problematic Variability in Results
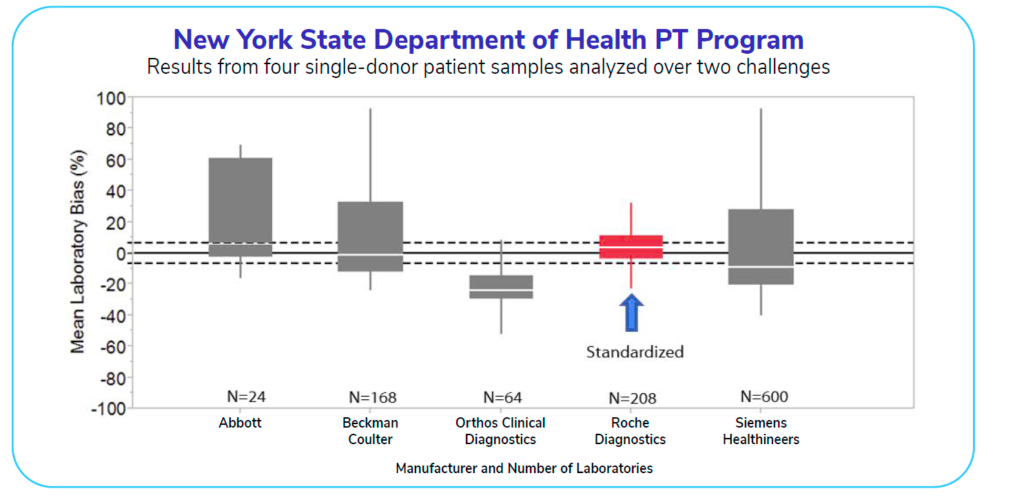
The graph below shows a study conducted in 2017 that displays assay manufacturers and the values from patient samples for testosterone tests. Only one manufacturer at the time was standardized for testosterone testing. This lone standardized test (displayed in red) has the greatest accuracy and reliability (evidenced by the red box being within the allowable measurement bias indicated by the dashed line). All of the assays above are FDA-approved, but only the standardized test is sufficiently accurate.
How many labs are currently using tests like the red one shown in the graph? Today, about 40-50% of labs operate using tests that are CDC-standardized for testosterone.
Testosterone: An Important Hormone to Standardize
Why should we consider testosterone as an important hormone? There are a few reasons. A diagnosis based on low testosterone might trigger a clinician to treat someone for disease. Though a single low testosterone level does not meet conditions for treatment of hypogonadism (it requires repeated values), clinicians rely on these lab tests as vital indicators.
In effect, clinicians could (and do) over- and under- diagnose hypogonadism due to extreme variability in testosterone values. This means inappropriate treatment that could cause side effects for the patient. On the overall healthcare system, this translates to increased healthcare costs and inconsistent clinical practice guidelines—making it difficult to convert research findings into evidence-based patient care.
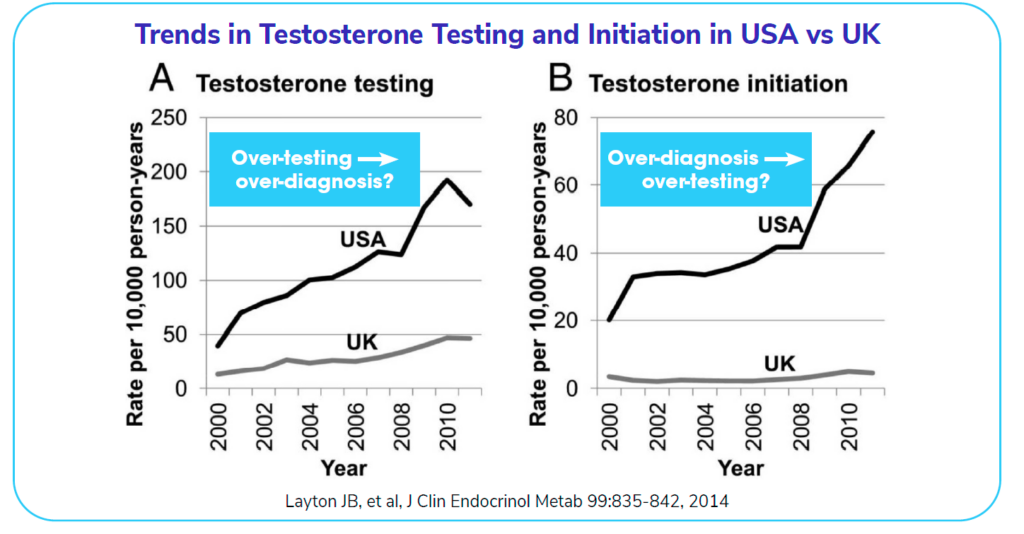
The following graph shows the trends in testosterone testing over the years. The difference between the USA and the UK on the left is striking. It reveals a remarkable amount of testosterone testing in the USA in comparison to the UK. Similarly, the over-diagnosis of testosterone-related issues (on the right) has trended up in the USA, while staying relatively flat in the UK. One possible reason for such large increases irrespective of the consistent use of standardized assays is the marketing of testosterone-related products to men in the USA.
The CDC Seeks to Ensure Tests Are Accurate, Reliable, and Comparable
How did the CDC develop its standardization program? The agency initiated its program by establishing reference values. These reference materials were then used and made available to manufacturers and laboratories to assess their analytical performance. Then, laboratories that perform within the desired specifications can become certified. Lastly, the CDC seeks to continuously monitor these test results at the patient care level to make sure the standard is consistently met.
The importance of the CDC program can be summarized in the following objectives:
- Accurate: Test calibration is appropriate and verified to be comparable to the CDC reference standard
- Reliable: Analytical precision is tested to meet clinical needs
- Comparable: Measurements in the same sample are similar in different tests over time Over time, the CDC has confirmed those assays that meet the standards for CDC standardization also have less variability and are more accurate.
Moving Forward: The Endocrine Society and Advocating for Increased Standardization
The Endocrine Society reference ranges can now be used with confidence as the ranges are linked to the CDC program. Clinical Practice Guidelines from the Endocrine Society similarly meet high standards.
Though FDA-cleared tests may not be sufficiently accurate (because the assays used by labs may not have met CDC rigorous performance requirements), providers can be assured that those labs certified by the CDC meet certain requirements. Standardization is a voluntary process, and more education is needed so that standardized and non-standardized tests are no longer used without distinction in clinical practice.
ADDITIONAL REFERENCES
The Partnership for the Accurate Testing of Hormones (PATH) – www.hormoneassays.org
CDC, Standardizing Hormone Measurements – https://www.cdc.gov/labstandards/pdf/hs/HoSt_Brochure.pdf
Endocrine Society, CME webinars for physicians – https://education.endocrine.org/content/accurate-hormone-testing-module-1-importance-hormone-measurements-and-assay-standardization
Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe, Journal of Clinical Endocrinology and Metabolism – https://academic.oup.com/jcem/article/102/4/1161/2884621
Laboratory Quality Assurance and Standardization Programs – https://www.cdc.gov/labstandards/index.html
Bhasin S, et al, J Clin Endocrinol Metab 103:1715-1744, 2018; Mulhall J, et al, J Urol 200:423-432, 2018; Le M, et al, J Urol 195:1556-1561, 2016
Avalon is the world’s first Lab Insights company pioneering a new era of value-driven care by unlocking the potential of lab results to generate actionable lab-driven insights in real time to proactively ensure appropriate care and enhance clinical outcomes. You can learn more about our impact here and the latest news here.
For more information, please contact us:
Barry S. Davis, Chief Growth Officer
201-218-3425 | [email protected]
Fred Barry, VP, Business Development
714-615-1889 | [email protected]
Rourke Day, Dir., Business Development
973-903-2809 | [email protected]
Jaclyn Ivonnet, VP, Business Development
407-758-2634 | [email protected]
Sara Sabin, VP, Business Development
845-591-4725 | [email protected]
AUGUST 26TH, 2-3 PM EST
- Medical Necessity & Prior Authorization
- Access, Equity & Value-Based Arrangements
- Build Ideal Prior Authorization Framework
- Anti-Kickback Statute Safe Harbor for Labs
With the persistence of the COVID-19 pandemic, lab-related policy continues to be pushed into the forefront of national media and policy discussions at every level of government. The time is ripe for our community of experts to engage. We hope you can join us as we discuss policy and strategy to guide us moving forward.
SPACE IS LIMITED! If you’d like to join us a member of the LAB Coalition team will be in touch with additional details.

AVALON WEBINAR: DELIVERING VALUE-DRIVEN CARE USING LAB TEST RESULTS
Tuesday, September 14 | 2:00 PM - 3:00 PM EDT
During this informative webinar, you’ll learn:
- What can be done today to lower total heathcare costs and optimize clinical outcomes
- How the right intel can support the early detection of disease
- Benefits of lab result-informed performance reporting
EXCERPT FROM THE 2021 AVALON LAB TREND REPORT
COMPLIANCE THROUGH AUTOMATED POLICY ENFORCEMENT
The automated policy enforcement program is a post-service, pre- payment solution that implements Avalon’s proprietary clinical lab editing. Using over 2M unique rules extracted from scientific-based coverage criteria, Avalon’s software provides real-time support for each payer’s policy decisions to approve claim lines, reduce them, or categorize them as non-complaint. When making a recommendation other than for approval, Avalon references the applicable policy used to make the determination. While policy enforcement does not alter members’ testing trajectory directly, it enables the plan to detect inappropriate and/or non-compliant testing, and to make payment adjustments accordingly. Furthermore, the policy guidance and provider education provided by the program when making adverse determinations helps to change physicians’ ordering behaviors and rendering laboratories’ order menus or laboratory panels. Since this solution is based on the science, there is no physician abrasion, and less than 1% of claims become reconsiderations.

Available Now: Industry’s First Lab Trend Report.
AVALON LABORATORY NETWORK COVID-19 CAPACITY & TURNAROUND TIME REPORT*
| Lab | Multiple Platforms | Capacity (per day) | TAT |
|---|---|---|---|
| Quest | Y | 300,000 | 1-2 days |
| LabCorp | Y | 275,000 | 1-2 days |
| Mako Medical Lab | Y | 150,000 | 1-2 days |
| Aegis | Y | 110,000 | 1-2 days |
| BioReference | Y | 100,000 | 1 day |
| Premier Medical Lab | Y | 100,000 | 1-2 days |
| Eurofins-Diatherix | N | 60,000 | 1-3 days |
| GenetWorx | Y | 40,000 | 2 days |
| AIT (American Institute of Tox) | Y | 20,000 | 1-2 days |
| PathGroup | Y | 20,000 | 1-2 days |
| Sonic-CPL | Y | 20,000 | 1-3 days |
| Genesis DX | Y | 16,000 | 1-2 days |
| MDL(Medical Diagnostic Lab) | N | 12,000 | 1 day |
| AccuReference | N | 10,000 | 2 days |
| LabTech | Y | 10,000 | 2 days |
| Inform Diagnostics | N | 5,000 | 1-2 days |
| Luxor | Y | 5,000 | 1 day |
| Neogenomics | Y | 5,000 | 1-4 days |
| Transplant Genomics | N | 5,000 | 1-2 days |
| Precision Genetics | N | 4,000 | 1-2 days |
| BAKO | N | 2,500 | 1-2 days |
| Radeas | Y | 2,400 | 1-2 days |
| NephronPharm | Y | 2,000 | 2-3 days |
| Wake Medical Lab Consultants | Y | 1,500 | 1 day |
| Andor Labs | N | 500 | 1-2 days |
| SMA | Y | 500 | 1-2 days |
*As of August 6, 2021

